人参皂苷
全网最全汇总!稀有人参皂苷的功效与作用
一、抗癌
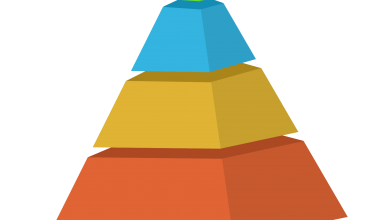
人参皂苷Rg3通过抑制EGFR-TPK和DNA拓扑异构酶I活性,降低膀胱癌细胞中VEGF、HIF-1α等促血管生成因子的表达(IC50=35.11 μM)。
Rh2:TNF-α介导的凋亡诱导
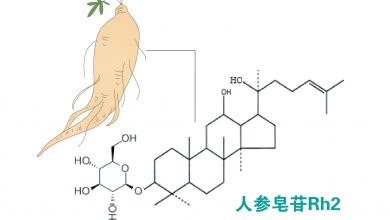
人参皂苷Rh2通过上调TNF-α激活caspase级联反应,诱导白血病细胞凋亡(HL-60细胞凋亡率达40%),对前列腺癌、三阴性乳腺癌等也显示剂量依赖性抑制。
人参皂苷Rk1通过抑制hTERT和c-Myc降低肝癌细胞端粒酶活性,同时激活caspase-8/3诱导凋亡。最新研究发现,Rk1通过抑制FSP1酶促发铁死亡,为高GPX4表达肿瘤提供新治疗策略。
二、心血管与代谢调节
预处理人参皂苷Rh3可降低大鼠血清LDH和CK水平(P<0.05),上调Bcl-2/Bax比值,抑制p38 MAPK/caspase-3通路,减少心肌细胞凋亡。
人参皂苷Rh4通过提升SOD和过氧化氢酶活性,缓解顺铂诱导的肾损伤,减少脂质过氧化物积累。在非酒精性脂肪肝模型中,Rh4下调TNF-α/IL-6,抑制NF-κB通路,改善脂代谢紊乱。
三、神经保护与认知改善
人参皂苷Rg5通过抑制PI3K/Akt/mTOR通路,同时激活食道癌细胞凋亡和自噬。在阿尔茨海默病模型中,Rg5减少β淀粉样蛋白沉积,调节胆碱能系统。
人参皂苷Rh1通过激活PI3K/Akt信号,降低β淀粉样蛋白诱导的氧化应激,恢复SH-SY5Y神经元活性。其与Rg2协同抑制LPS诱导的炎症因子释放,保护肝肾。
四、免疫调节与抗炎作用
人参皂苷Rk2通过SIRT1介导的ERK/MEK通路去磷酸化,抑制溃疡性结肠炎中NLRP3炎症小体,并修复肠道紧密连接蛋白。
人参皂苷Rk3在食管癌中抑制PD-L1表达,同时增强NK细胞活性,实现免疫微环境的重编程。
五、代谢调控与化疗增敏
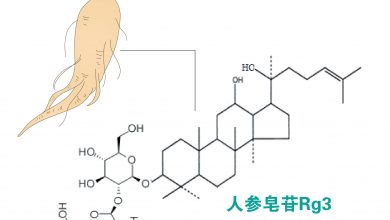
人参皂苷aPPD通过干扰脂质筏中Akt信号,增强化疗药物敏感性。在前列腺癌中,aPPD抑制雄激素受体N端域,降低PSA水平达53%。
人参皂苷aPPT作为原人参三醇的最终代谢产物,通过β-糖苷酶完全转化,显著提高生物利用度,调节能量代谢。
六、多组分协同:1+1>2的增效密码
研究证实,人参皂苷的协同作用源于多通路交叉调控:
- 信号通路互补:如人参皂苷Rg3抑制PI3K/Akt的同时,Rh2激活TNF-α通路,形成抗癌网络。
- 代谢增效:人参皂苷Rb1与Rg1组合可同时调节线粒体复合物I和SIRT1,增强心肌保护。
- 生物利用度提升:稀有人参皂苷(如Rk3、Rh4)通过发酵或酶解转化,其活性较原型皂苷提高3-5倍。
单一成分难以覆盖复杂疾病网络,多组分人参皂苷制剂可通过”多靶点、低毒性”的优势,为癌症、神经退行性疾病等提供更优解决方案。
从抗癌前线到神经保护,人参皂苷的多元作用正重新定义天然药物的价值。

目前,多组分人参皂苷产品瑞得生胶囊(Redsenol-1 Plus)正在加拿大开展癌症相关疲劳的临床试验,该产品正式被美国国家癌症研究所(National Cancer Institute, NCI)药物词典收录,有望进一步推动多组分人参皂苷标准化制剂的临床应用,让千年人参焕发现代医学的光彩。
参考资料:
1. S. Nag, Jiangjiang Qin et al. “Ginsenosides as Anticancer Agents: In vitro and in vivo Activities, Structure–Activity Relationships, and Molecular Mechanisms of Action.” Frontiers in Pharmacology(2012). 2. Wei Liu, Sheng-Xiong Zhang et al. “Ginsenoside Rg3 Promotes Cell Growth Through Activation of mTORC1.” Frontiers in Cell and Developmental Biology(2021). 3. M. Endale, Wm Lee et al. “Ginsenoside‐Rp1 inhibits platelet activation and thrombus formation via impaired glycoprotein VI signalling pathway, tyrosine phosphorylation and MAPK activation.” British Journal of Pharmacology(2012). 4. Jong Dae Park, D. Rhee et al. “Biological Activities and Chemistry of Saponins from Panax ginseng C. A. Meyer.” Phytochemistry Reviews(2005).. Springer. 5. Taeui Hong, M. Kim et al. “Ca2+-activated mitochondrial biogenesis and functions improve stem cell fate in Rg3-treated human mesenchymal stem cells.” Stem Cell Research & Therapy(2020). 6. Xiao-huang Xu, Ting Li et al. “Saponins from Chinese Medicines as Anticancer Agents.” Molecules(2016).. Xiao-Huang Xu, Ting Li. 7. M. Nakhjavani, Eric Smith et al. “Anti-Angiogenic Properties of Ginsenoside Rg3.” Molecules(2020).. Maryam Nakhjavani, Eric Smith. 8. Siying Teng, Yi Wang et al. “Effects of R type and S type ginsenoside Rg3 on DNA methylation in human hepatocarcinoma cells.” Molecular Medicine Reports(2017).. SIYIN. 9. M. Nakhjavani, J. Hardingham et al. “Ginsenoside Rg3: Potential Molecular Targets and Therapeutic Indication in Metastatic Breast Cancer.” Medicines(2019). 10. Junho Lee, Ha-Yeon Lee et al. “Enhanced Minor Ginsenoside Contents of Nano-Sized Black Korean Ginseng through Hot Melt Extrusion.” Materials(2024). 11. Hyeon-Muk Oh, Chong-kwan Cho et al. “Experimental Evidence for the Anti-Metastatic Action of Ginsenoside Rg3: A Systematic Review.” International Journal of Molecular Sciences(2022). 12. Sun-Hye Choi, Byung‐Hwan Lee et al. “Ginseng Gintonin Activates the Human Cardiac Delayed Rectifier K+ Channel: Involvement of Ca2+/Calmodulin Binding Sites.” Molecules and Cells(2014).. Mol. Cells. 13. S. Nah. “Ginseng ginsenoside pharmacology in the nervous system: involvement in the regulation of ion channels and receptors.” Frontiers in Physiology(2014). 14. Chan Joong Kim, Bomi Kim et al. “Variations in Ginsenosides of Raw Ginseng According to Heating Temperature and Time.” Journal of Pharmacopuncture(2020).. Chan Joong Kim. 15. H. Vo, J. Cho et al. “Kinetic study for the optimization of ginsenoside Rg3 production by heat treatment of ginsenoside Rb1.” Journal of Ginseng Research(2015). 16. Mao-xiu Peng, Gangyl Jiang et al. “Effects of ginsenoside Rg3 on bone loss, bone mineral density and osteoclast number in glucocorticoid-induced osteoporosis rats, and the likely michanism of action.” Tropical Journal of Pharmaceutical Research(2020). 17. Yang, L., Zhang, Xy., Li, K. et al. Protopanaxadiol inhibits epithelial–mesenchymal transition of hepatocellular carcinoma by targeting STAT3 pathway. Cell Death Dis 10, 630 (2019). 18. H. Fan, Sun Xiao-ling et al. “Comparative Pharmacokinetics of Ginsenoside Rg3 and Ginsenoside Rh2 after Oral Administration of Ginsenoside Rg3 in Normal and Walker 256 Tumor-bearing Rats.” Pharmacognosy Magazine(2016).
除非注明,否则均为瑞得生健康网原创文章,转载必须以链接形式标明本文链接
本文链接:https://redsenol.com/41294.html


_副本-390x220.jpg)




_副本-390x220.jpg)








发表你的观点